Application Note – Bioaerosol N.13 – Microbiological Air Monitoring in Operating Room

Introduction
The prime function of a “Ventilation System” or a “Heating Ventilation Air Conditioning System” (HVAC) in an operating theatre, intensive treatment units and isolation suites is to closely control the environment and air movement of the space that it serves in order to contain, control and reduce hazard to patients and staff from airborne contaminants, dust and harmful micro-organisms.
A “Ventilation System” in itself present no danger to patient and staff; however it possesses the ability to transmit hazards arising from other sources to large number of people. The basic fact is that the danger may not become apparent until many patients and staff have been affected.
Patients and staff have a right to expect that a “Ventilation System” will be designed, installed, operated and maintained to standards that will enable it to fulfil its desired functions reliably and safely.
Material
- Microbiological air sampler suitable for remote activation
- Floor tripod
- Contact Plate with Plate Count Agar
- Spray disinfectant
Protocol
We report the original text of the Official Document written by the U.K. Health Department “Health Technical Memorandum 2025 – Validation and Verification – Ventilation in Healthcare Premises - HMSO”.
Operating rooms (Monitoring of empty premises)
5.33 The level of airborne bacteria introduced by the supply air can be checked by closing all doors and leaving the operating room empty with the ventilation system running for one hour, after which a bacterial sampler mounted on the operating table should be activated remotely.
Aerobic culture on non-selective medium should not exceed 35 bacterial and or fungal particles per cubic metre of ventilating air.
5.34 The results should be examined to establish the broad category of organisms present. A high preponderance of fungal organisms may be an indication of inadequate filtration for the particular installation.
Operating rooms (Monitoring during a surgical operation)
5.35 A check of airborne bacteria during a surgical operation should be carried out as soon as possible after handover.
Unless there are unusually high numbers of personnel or extensive activity in the room, the number of airborne bacterial and/or fungal CFUs (colony forming unit) averaged over any five minute period, should not exceed 180 per cubic metre.
This work should be carried out by the same nominated infection control officer or consultant microbiologist if not the same person.
Ultra-clean systems (Monitoring during surgical procedures)
5.36 The installation should be tested during surgical procedures at intervals between the time of first incision and final closure of the wound.
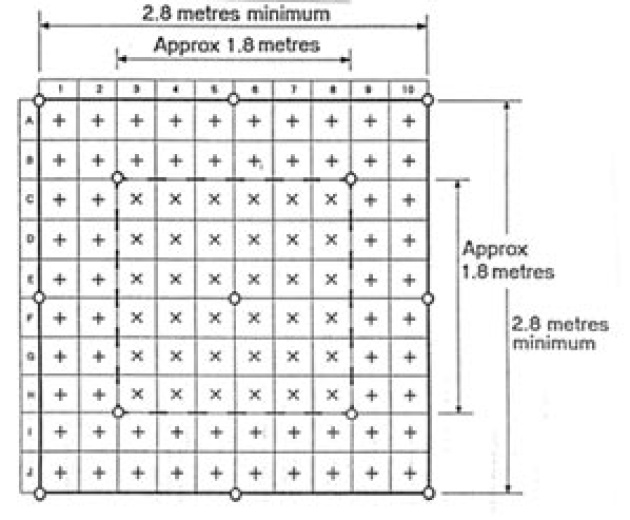
A test grid is shown in Figure:
Bacteriological Sampling Position
+ Measure air velocity 2m above floor level
x Measure air velocity 2m above floor level and also measure air velocity 1m above floor evel (min 0.2 m/s)
O Bacteriological sampling position
The following minimum performance requirement should be obtained:
a. air leaving the diffuser or final filters should contain not more than 0.5 CFUs/m3 of air. If the air filters have been tested after installation by a particle penetration test, this test is not necessary;
b. air sampled close to the wound site during operations, that is within 300 mm of the wound on average, contain less than 10 CFUs/m3 of air using conventional cotton clothes. Level less than 1 CFUs/m3 can be expected when using occlusive clothing or body exhaust systems;
c. air sampled at the perimeter of the clean zone during surgery should contain not more than 20 CFUs/m3 using conventional clothing and levels less than 10 CFUs/m3 when using occlusive clothing or body exhaust systems.
Reference
Health Technical Memorandum 2015.